Nice Tips About How To Tell If Bonds Are Polar
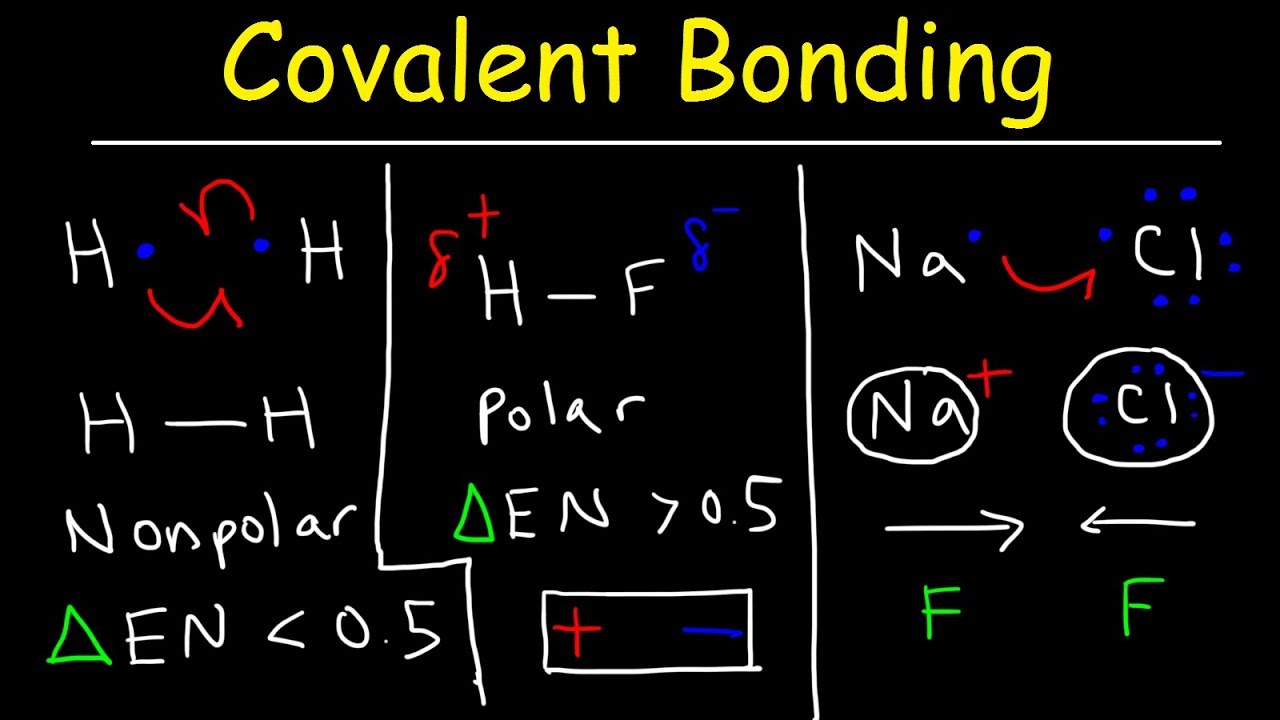
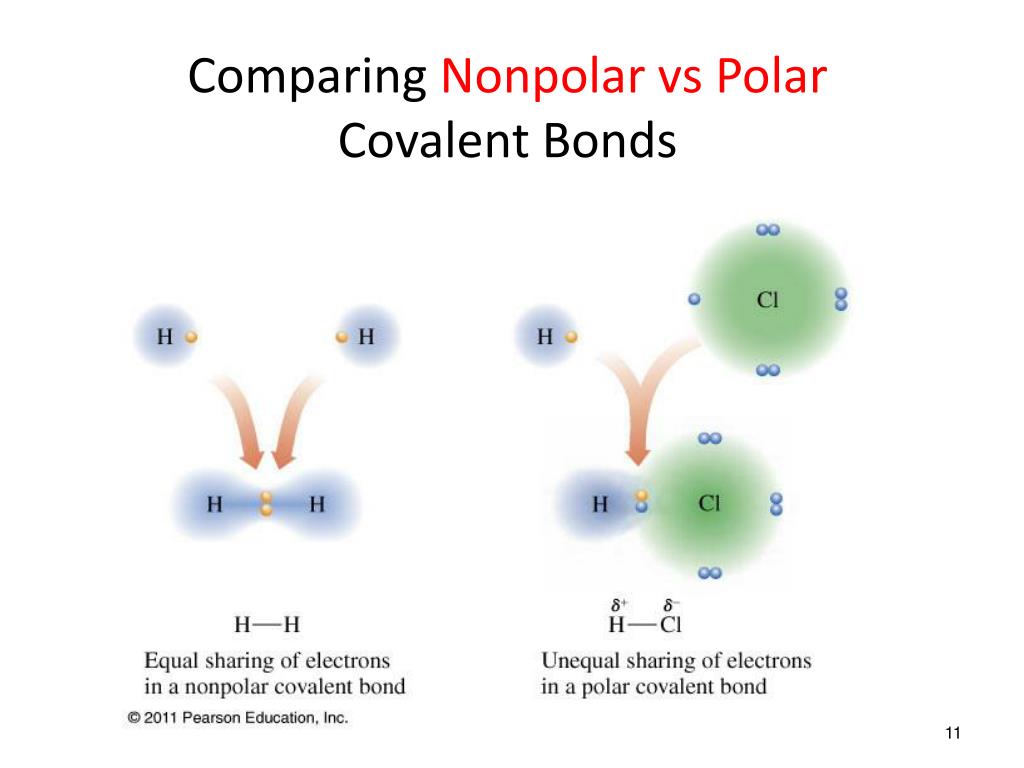
A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so.
How to tell if bonds are polar. 2.5m views 8 years ago. The difference is how the electrons in the bond are arranged. Result when determining molecule polarity, all bonds must be considered.
287k views 9 years ago. Chemical bonds may be classified as being either polar or nonpolar. A bond distance (or bond length) is the distance.
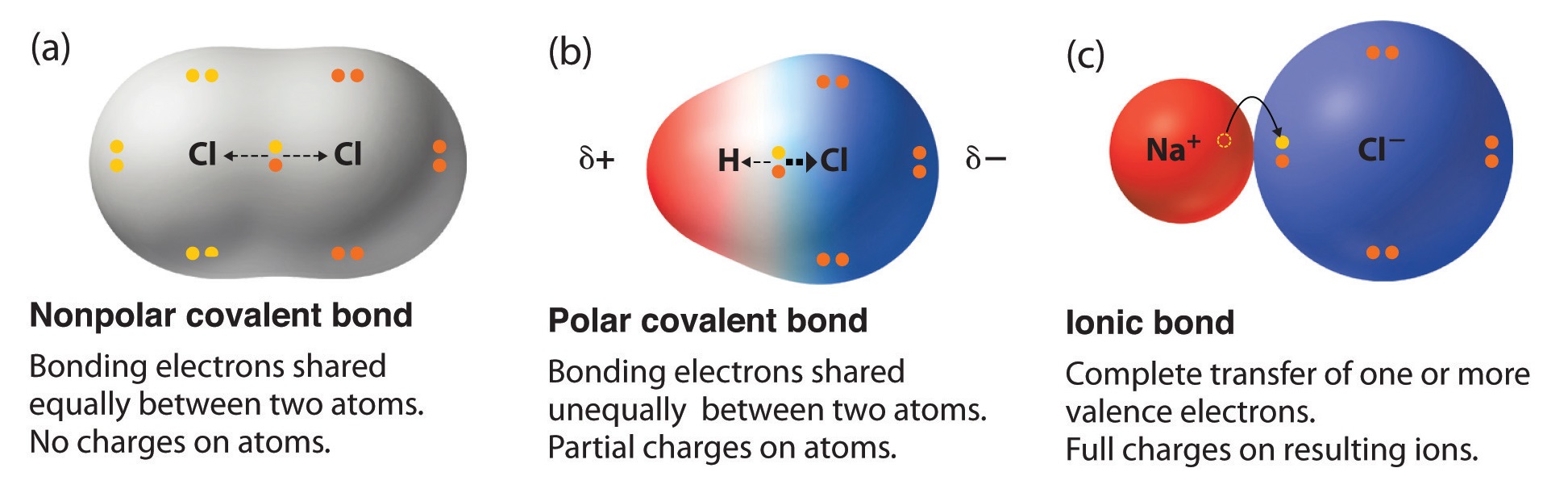
Result their bond polarity is determined according to the range it falls in: Classifying bonds as covalent, polar covalent, or ionic. Result written by sushmita rout.
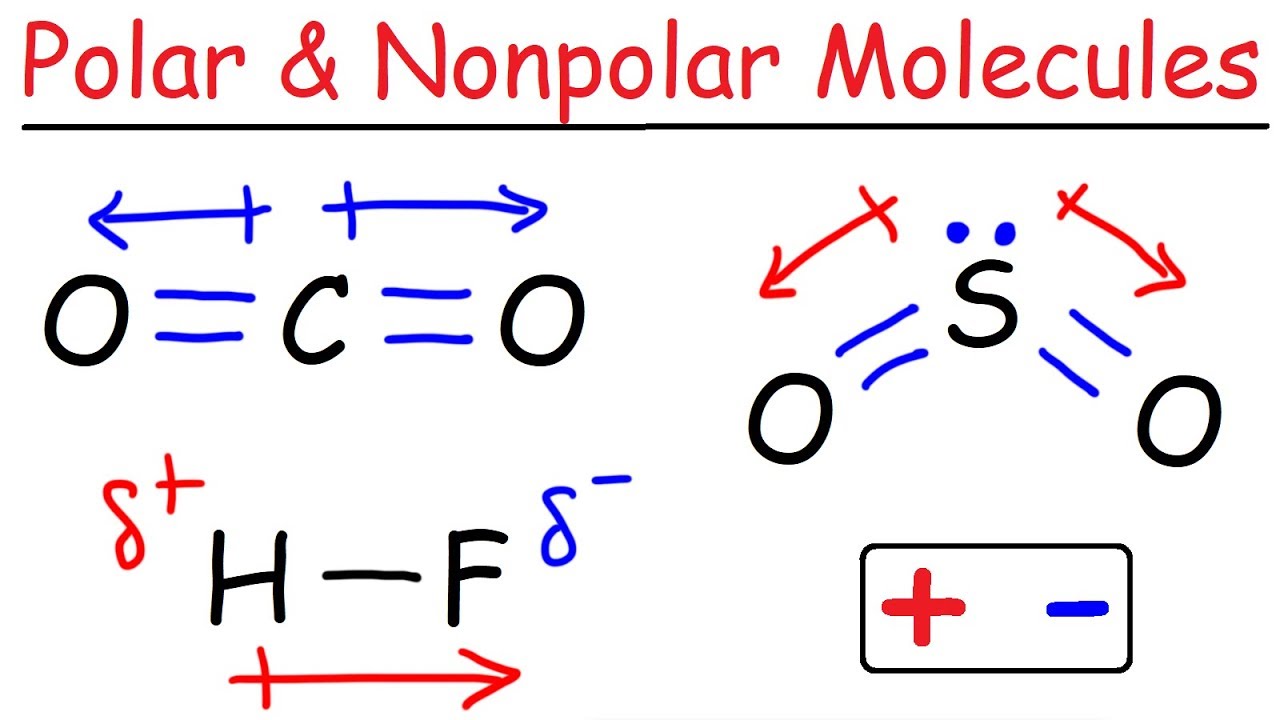
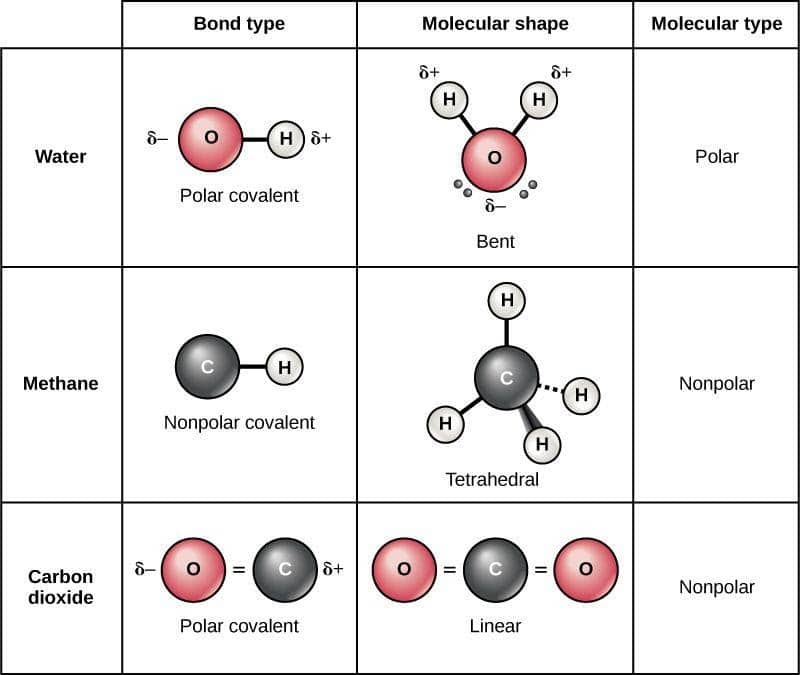
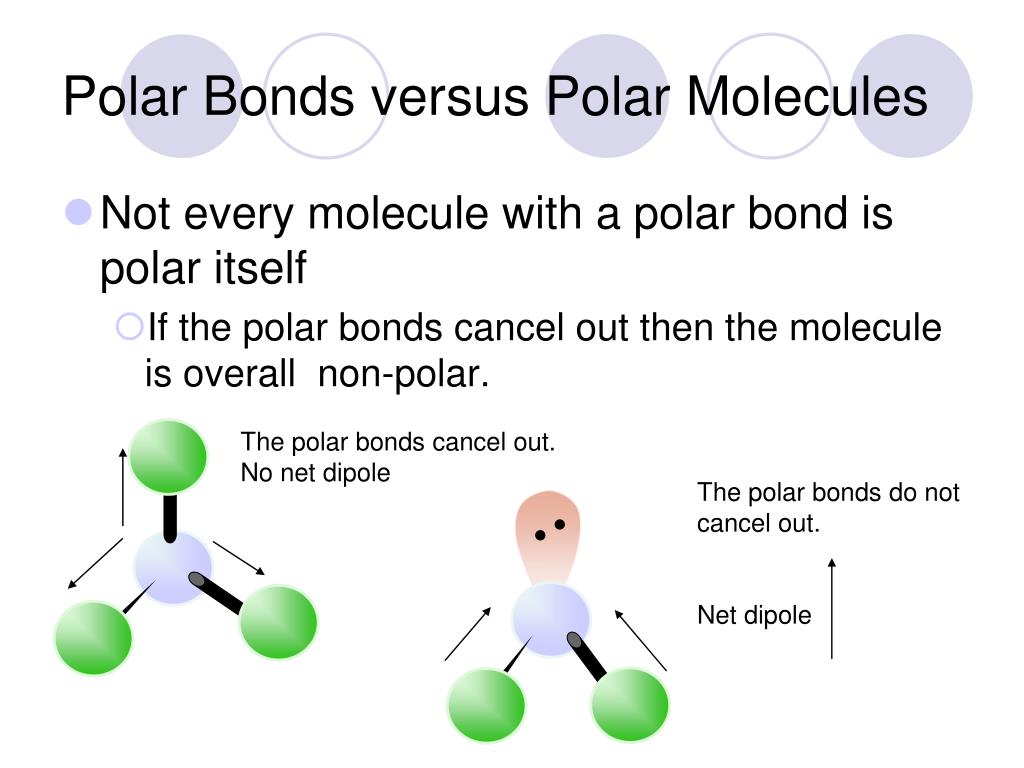
This means that the vector partial charge from each bond must be added up. Result both of the bonds inside the molecule are polar bonds. This video provides a fast way for you to determine if a molecule is.
In short the molecular dipole moment is the vector sum of the individual. Bond distances are measured in. When evaluating the polarity of a molecule, the.
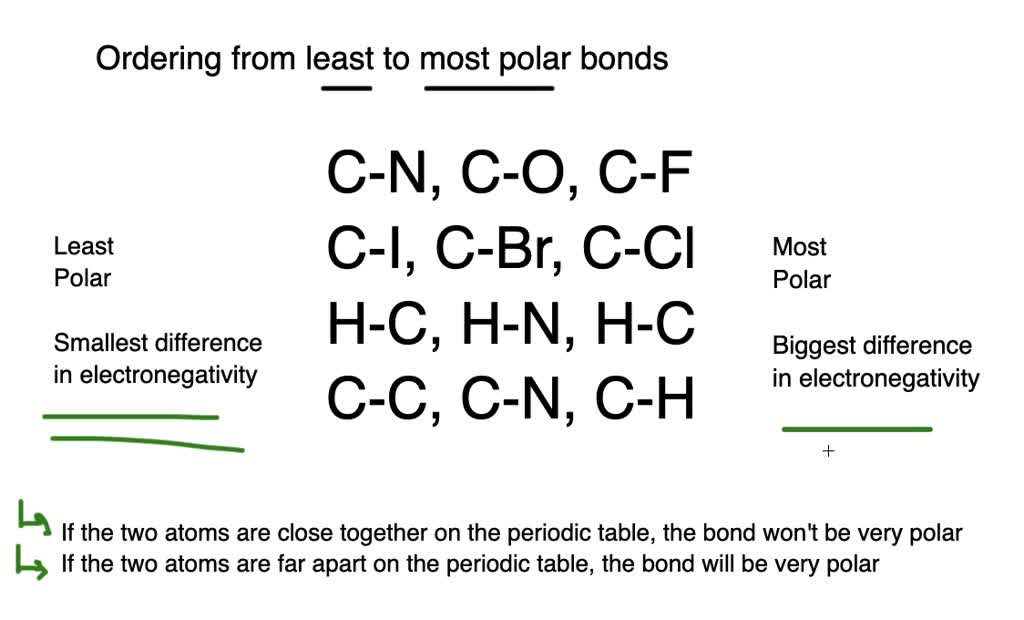
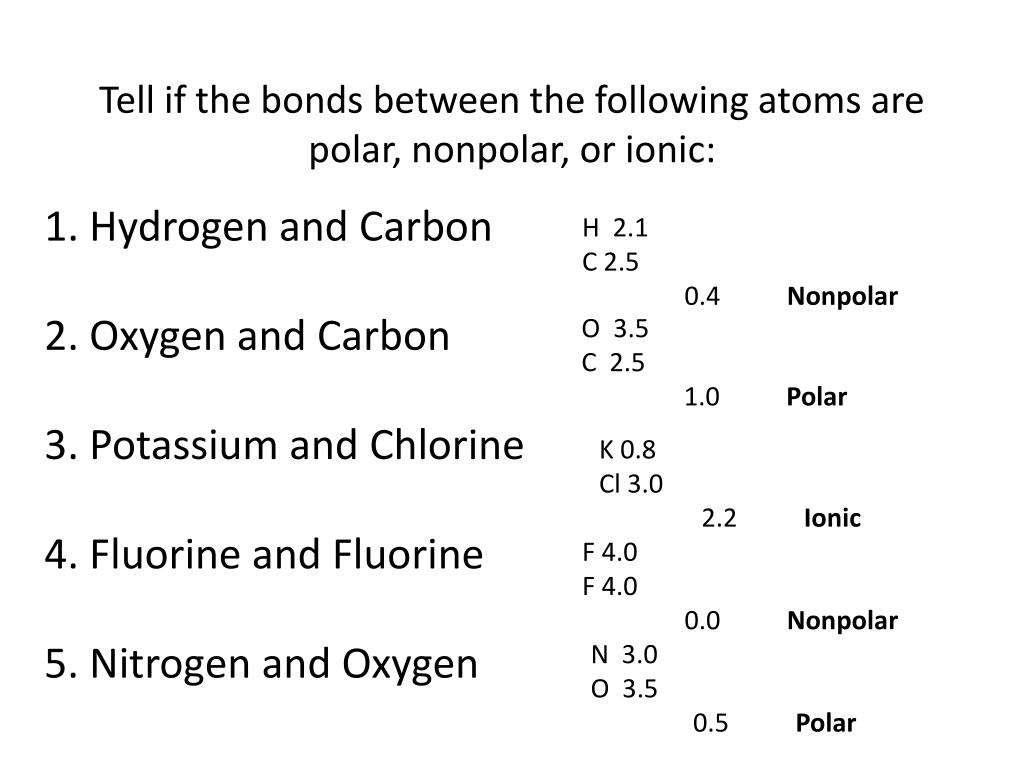
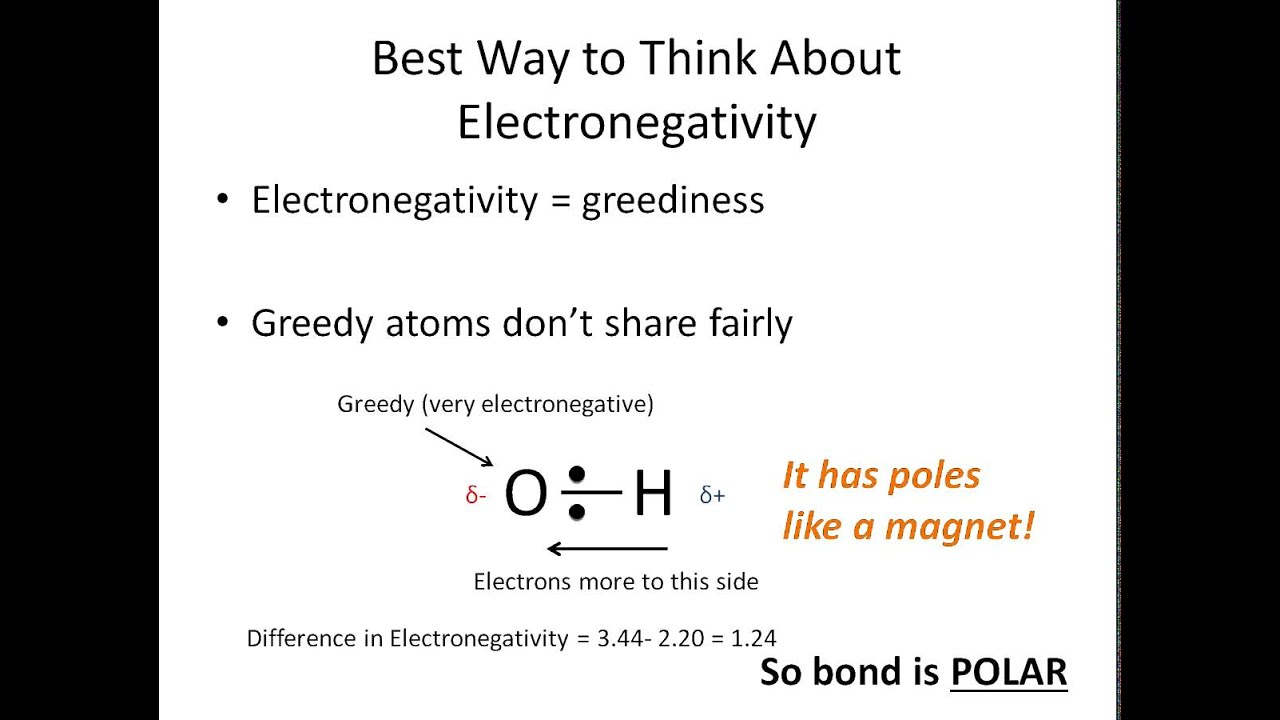
Result like bonds, molecules can also be polar. Electronegativity differences in bonding using the pauling scale. Result the bond polarity between two atoms can be estimated if you know the electronegativity of both elements.
Result a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. Result how to tell if a bond is polar or nonpolar (the super easy way) the complete guide to everything. Result updated on april 01, 2021.
Result it is a measure of an atom's ability to attract and hold onto electrons within a chemical bond. Bond polarity and molecular polarity are different (though related) concepts. Result a diatomic molecule that consists of a polar covalent bond, such as \(\ce{hf}\), is a polar molecule.
Result the organic chemistry tutor. Result a bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. When two atoms share a pair of.





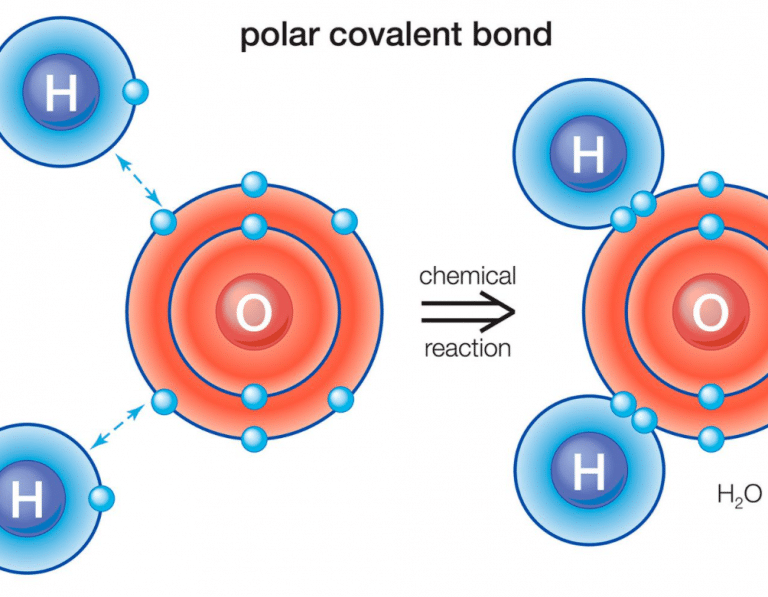



:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)